A New Approach to Therapeutic Drug Monitoring of Tobramycin in Cystic Fibrosis
In this article:
EXECUTIVE SUMMARY
INTRODUCTION
Intravenous (IV) tobramycin, an aminoglycoside antibiotic, is a standard treatment for Pseudomonas infection in patients with cystic fibrosis (CF). However, drug exposure must be maintained within a narrow therapeutic range. If the dose is too high, there is an increased risk of adverse events, such as nephrotoxicity and ototoxicity.1,2 If the dose is too low, there is an increased risk of therapeutic failure, antibiotic resistance and poor patient outcomes.3,4 As a result, therapeutic drug monitoring (TDM) – measurement of drug concentrations in the blood at known times – of tobramycin is essential to enable appropriate dose individualization.
KEY CHALLENGES OF CONVENTIONAL METHODS ESTIMATING AUC FOR TOBRAMYCIN
Dose individualization of tobramycin is usually performed by taking two blood samples at fixed time points (relative to dose administration) and interpolating
these into an estimation formula. This conventional method for estimating tobramycin area under the curve (AUC) – the actual body exposure to a drug after administration of a dose – is associated with a number of practical challenges, including difficulties surrounding drawing two blood samples, where patients often fail to present for their second blood test at the proposed time.5
CONCLUSION
In this study, DoseMe, a Bayesian dose individualization platform, is shown to be more accurate at estimating AUC than the conventional two-sample method while requiring only one blood sample and allowing the collection of this sample at almost any patient preferred time.

CURRENT CHALLENGES ASSOCIATED WITH
THERAPEUTIC DRUG MONITORING OF TOBRAMYCIN
THE CHALLENGE
To be effective and well tolerated, tobramycin drug exposure must be maintained within a narrow therapeutic range. Achieving this is further complicated by a high degree of inter- and intra-patient variability in the PK parameters of tobramycin. As a result, therapeutic drug monitoring (TDM) of tobramycin is essential to enable appropriate dose individualization beyond covariate-based dosing (i.e. using age and/or mg/kg).
TOBRAMYCIN DOSE TOO HIGH
Increased risk of nephrotoxicity
and ototoxicity, of which the
latter may be irreversible1,2
TOBRAMYCIN DOSE TOO HIGH
Increased risk of therapeutic
failure, antibiotic resistance
and poor patient outcomes3,4
WHAT IS THERAPEUTIC DRUG MONITORING?
TDM is the measurement of drugs at specific intervals to maintain a constant concentration in a patient’s bloodstream – to optimize individual dosage regimens. Performing TDM requires a multidisciplinary approach
that is integral to the clinical success of dose individualization for drugs with narrow therapeutic ranges and pharmacokinetic variability
CLINICAL RELEVANCE
Accurate and clinically meaningful drug concentrations are attainable only by collaboration across multiple departments, with most methods relying on multiple blood samples to be taken at specific times.7
In a busy and demanding clinical environment, the optimal collection period for TDM samples is short, and unsurprisingly, inaccurately timed blood collections are common. In one study, only 4.3% of blood samples were deemed to have been taken at the correct time.8
This increases the potential for misleading clinical decision making on these results, having implications for patient safety, including sub- or supratherapeutic dosing.
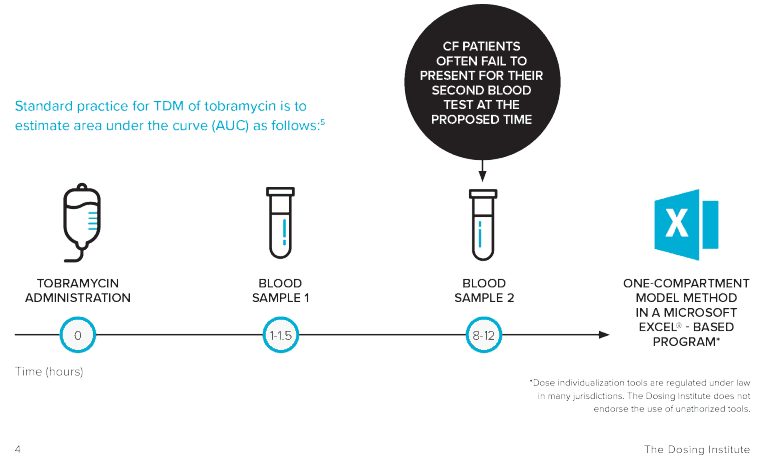
TOBRAMYCIN TDM STUDY OVERVIEW
AIM
To compare the conventional two-sample AUC estimation method with a one concentration Bayesian method* using DoseMe, and compare these with the true AUC, calculated from sampling across eight timepoints.
STUDY DESIGN
Data were collected from adult CF patients prescribed once-daily intravenous tobramycin at the Mater Health Service (MHS) Respiratory Unit, Brisbane, Australia.
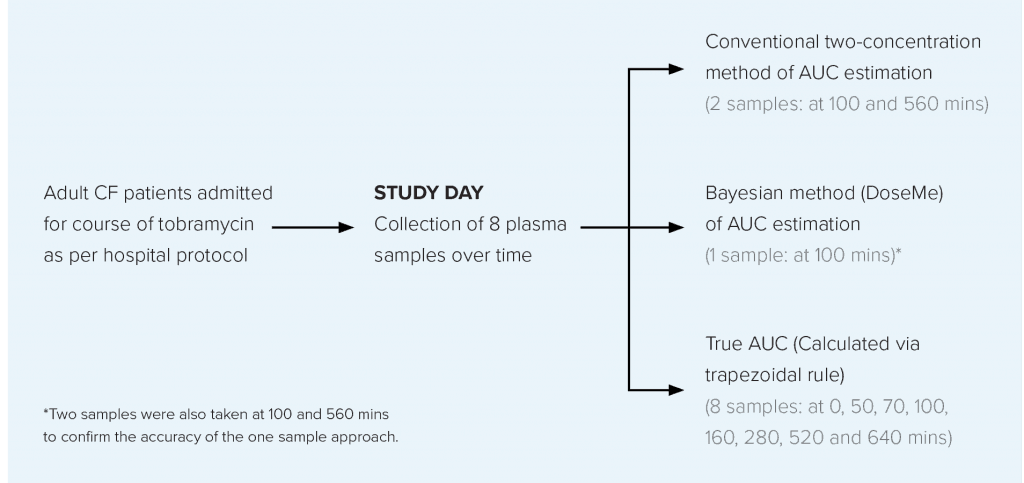
RESULTS
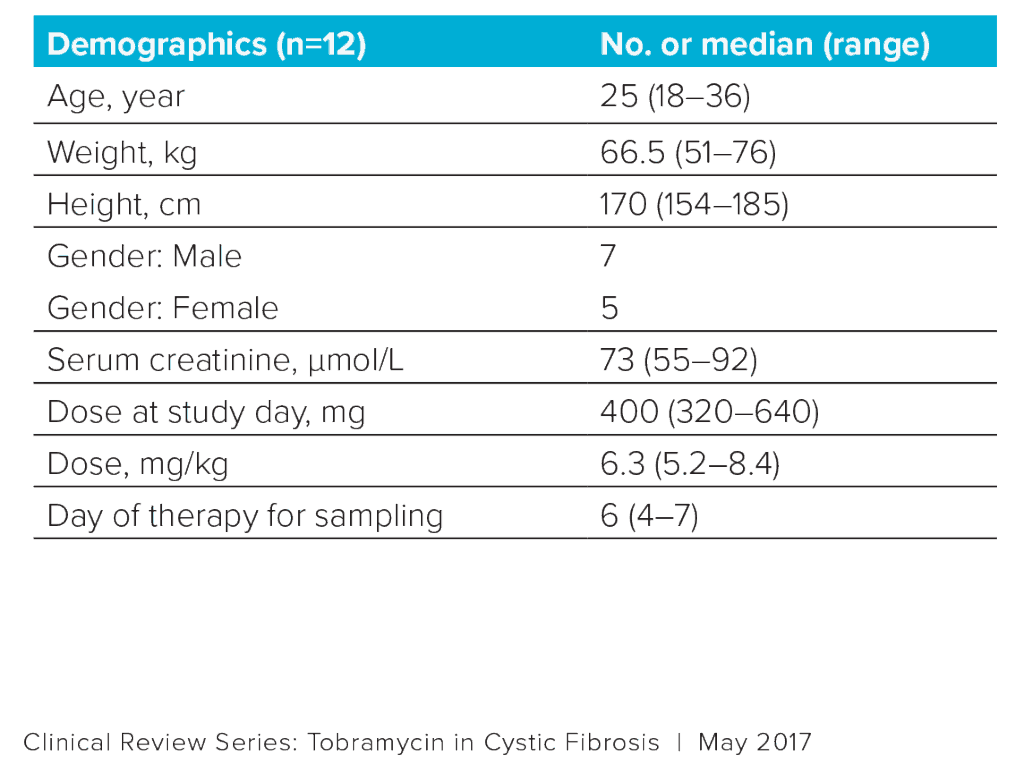
Data were collected from 12 patients with CF (Table 1).
*Bayesian dose individualization decision support systems, such as DoseMe, provide pharmacokinetic
model-based clinical decision support. These methods integrate patient data and laboratory results with a prior population pharmacokinetic and pharmacodynamic to estimate a patient’s ability to absorb, process and clear a drug from their system.
Bayesian dosing tools adjust the parameters so that a patient-specific, individualized drug model is created. This individual model is then used to
provide a patient-specific dosing recommendation to reach a specific target exposure.
With one sample, the Bayesian method using DoseMe provided similar estimated AUC values to the true, 8-sample trapezoidal AUC. Furthermore, the Bayesian method was both more precise and less biased than the two-sample conventional method using any one sample taken between 2-4 hours after initiation of tobramycin infusion.
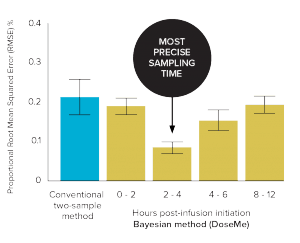
Figure 2. Precision (root mean squared error or RMSE) vs 8-sample trapezoidal method of the conventional two-sample and Bayesian one-sample methods
(DoseMe) up to 12 hours’ post-infusion initiation
The Bayesian method
(2–4 hours) using DoseMe
was significantly more precise
than the conventional method.
(RMSE 8% vs 21%, respectively)
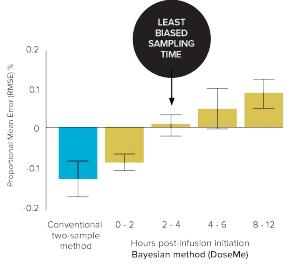
Figure 3. Bias (Mean Error) vs 8-sample trapezoidal method of the conventional
two-sample and Bayesian one-sample method using DoseMe up to 12 hours’ post infusion initiation.
The Bayesian method
(2–4 hours) using DoseMe
was significantly less biased.
(ME 0% vs – 14%, respectively)
THE BENEFITS OF A ONE-SAMPLE BAYESIAN METHOD FOR TDM OF TOBRAMYCIN
CONCLUSION
This study demonstrates that tobramycin TDM in CF patients can be simplified safely with a one-sample Bayesian method. The reduced requirement for blood collection samples not only lessens the burden placed on CF patients by alleviating overall time spent in hospital, but additionally reduces cost by removing unnecessary assays.
Overall, this study shows that using a one-sample Bayesian method such as DoseMe, reduces the restriction on the sampling times, which is known to be problematic in the hospital environment. By allowing the use of any sampling time, Bayesian methods reduce the likelihood of inappropriate use of assays, assisting healthcare practitioners to select the most appropriate dose to achieve an optimal response and reduce potential toxicity
DoseMe using a one-sample
Bayesian dosing method achieves higher accuracy and precision in calculating AUC using half as many laboratory results as the conventional clinical method.
Using the DoseMe Bayesian dosing method, only one assay result is required for accurate AUC estimation.
DoseMe using a one-sample
Bayesian method allows collection to be at a patient or provider preferred time. While ideally 2-4 hours after initiation of infusion – any single time up to 12 hours is as accurate as conventional methods.
REFERENCES
- Ratjen F et al. J Cyst Fibros 2009;8:361–9.
- Mulheran M et al. Antimicrobial Agents Chemotherapy 2001;45:2502–9.
- Cohen-Cymberknoh M et al. Am J Respir Crit Care Med 2011;183:1463–71.
- Ratjen F, McColley SA. Am J Respir Crit Care Med 2012;185:933–6.
- Barras MA et al. Antimicrobial Agents Chemotherapy 2016;60:6698–702.
- Mould G et al. Clinical Pharmacology & Therapeutics 2016;99/4:405-418.
- Hennig S et al. Clinical Pharmacokinetics 2013; 52:289-301.
- Li C, Therapeutic Drug Monitoring of Vancomycin in the real world. IATDMCT Compass. Mar 2017.
Try DoseMeRx – Free Trial
Try DoseMeRx for yourself, with a free 14 day trial including your choice of drug packages from a wide range of medications, customize dose or target functions to individualize treatment for every patient, and access our mobile and tablet apps for iOS and Android. During your free trial, you’ll also have access to our support team who are here to answer any questions you have. Start free trial now >>