Therapeutic Drug Monitoring for Busulfan IV Dosing
In this article:
THE CURRENT LANDSCAPE
Intravenous (IV) Busulfan is an alkylating drug routinely used in conditioning regimens before allogenic haemopoietic cell transplantation (HCT), the standard treatment for various malignant and non-malignant disorders. However, drug exposure must be maintained within a narrow therapeutic range. If the drug exposure is too high, there is an increased risk of toxicity, such as mucositis, graft vs host disease, veno-occlusive disease, sinusoidal obstructive syndrome, as well as increased risk of transplantation-related mortality. If the dose is too low, there is an increased probability of graft rejection or disease relapse.1 As a result, therapeutic drug monitoring (TDM) – measurement of drug concentrations in the blood at known times – of busulfan is essential to enable appropriate dose individualization.

KEY CHALLENGES OF CONVENTIONAL METHODS
Dose individualization of busulfan is usually performed by taking between 5 and 8 blood samples at fixed time points (relative to dose administration) and estimating area under the curve (AUC) using the trapezoid method. Based on the calculated AUC, the dose is then adjusted, and the process is repeated several doses later, several times during the course of treatment. The conventional method for measuring AUC and adjusting the busulfan dose is associated with a number of practical challenges, including the need to draw between 5 and 8 blood samples every time AUC is measured, and the accuracy of the process used to adjust the dose.
CONCLUSION
In conclusion, when compared to the conventional eightsample method, this study demonstrated that DoseMeRx:
- is as accurate at estimating an AUC using only two blood samples,
- provides greater flexibility as to the timing of samples collected,
- is as accurate at predicting blood plasma concentration, and therefore calculating the next dose required to obtain an AUC in the desired therapeutic range.
CURRENT CHALLENGES ASSOCIATED WITH
THERAPEUTIC DRUG MONITORING OF BUSULFAN
THE CHALLENGE
To be effective and well tolerated, busulfan drug exposure must be maintained within a narrow therapeutic range. Achieving this is further complicated by a high degree of inter- and intrapatient variability in the PK parameters of busulfan. As a result, therapeutic drug monitoring (TDM) of
busulfan is essential to enable appropriate dose individualization beyond covariate-based dosing (i.e. using age and/or mg/kg.) 2,3
BUSULFAN DOSE TOO HIGH
Increased risk of toxicity and transplantation-related mortality
BUSULFAN DOSE TOO LOW
Increased risk of graft failure or disease relapse2,3,4
CLINICAL RELEVANCE
Accurate and clinically meaningful drug concentrations are attainable only by collaboration across multiple departments, with most methods relying on multiple blood samples to be taken at specific times
In a busy and demanding clinical environment, the optimal collection period for TDM samples is short, and unsurprisingly, inaccurately timed blood collections are common. In one study, only 4.3% of blood samples were deemed to have been taken at the correct time.8 This increases the potential for misleading clinical decision making on these results, having implications for patient safety, including subor supratherapeutic dosing.
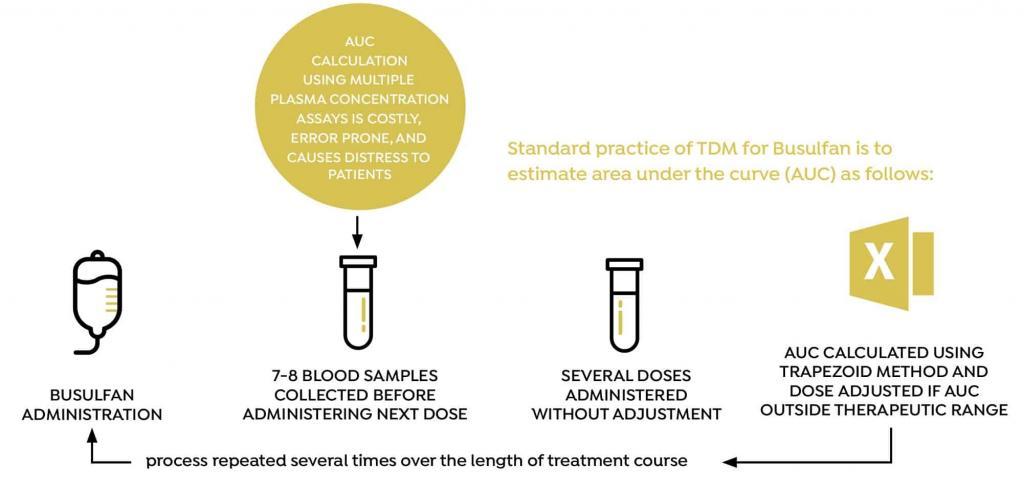
BUSULFAN STUDY OVERVIEW
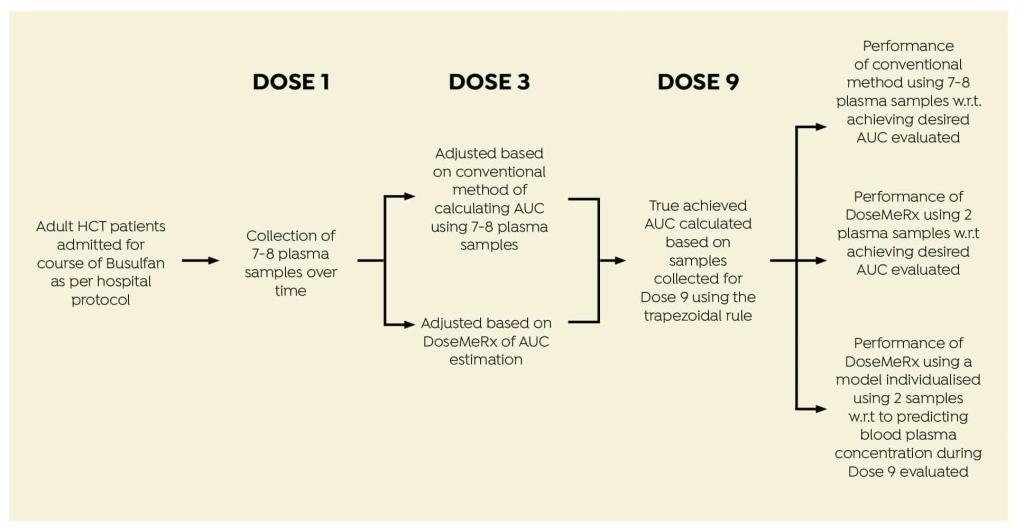
AIM
To compare the conventional multi-sample AUC estimation model with a two-sample protocol using DoseMeRx, and compare these methods with respect to achieving the AUC in the desired therapeutic range.
STUDY DESIGN
Data were collected from adult HCT patients given four times daily intravenous busulfan.
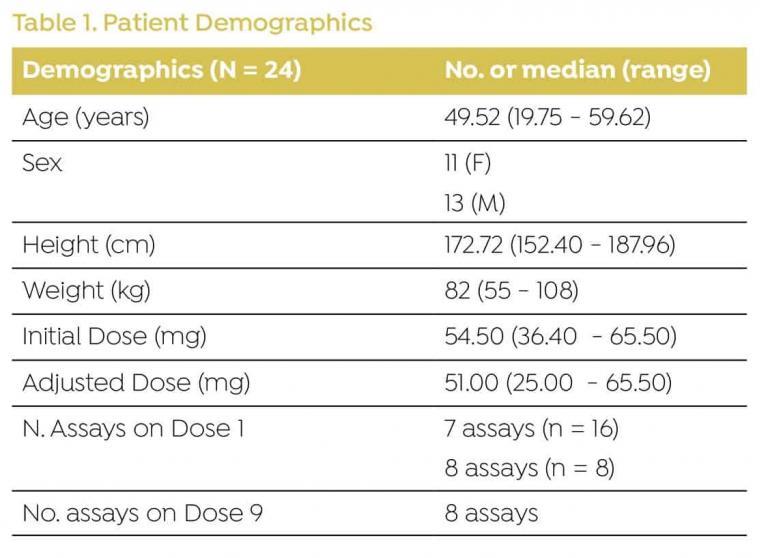
DEMOGRAPHICS
Data were collected from 24 patients admitted for HCT (Table 1).
*Dose individualization decision support systems provide pharmacokinetic model-based clinical decision support. Bayesian methods such as DoseMeRx integrate patient data and laboratory results with a prior population pharmacokinetic and pharmacodynamic model to estimate a patient’s ability to absorb, process and clear a drug from their system. DoseMeRx adjusts the parameters so that a patient-specific, individualized drug model is created. This individual model is then used to provide a
patient-specific dosing recommendation to reach a specified target exposure.
RESULTS
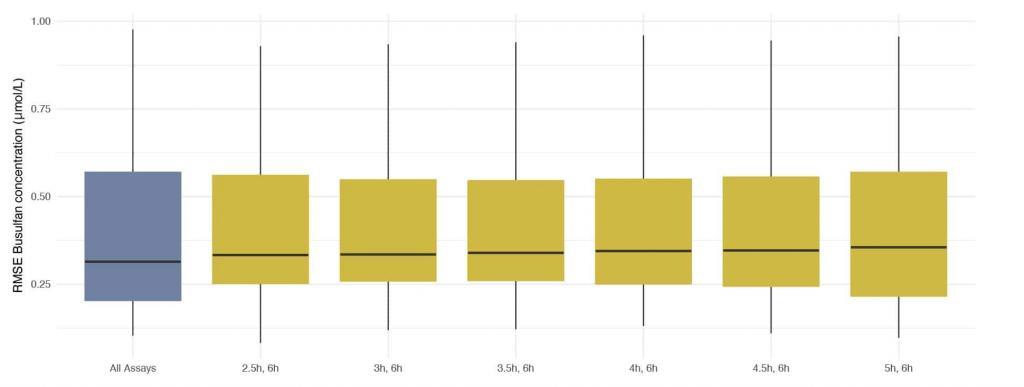
Assays taken at 2h (peak) post beginning of administration were excluded. Any combination of two assays that included a trough (assay taken 6h post administration and just prior to administration of the next dose) resulted in very low discrepancies between concentrations predicted using DoseMeRx and those measured by assays performed on Dose 9. The best combination of assays (lowest median RMSE) was that taken 2.5 and 6 hours after administration
Best combination of assays for model fitting
Authors’ Highlight:
DoseMeRx presents a clear opportunity to reduce the number of assays performed by 75% – improving operational efficiency without compromising patient outcomes. A trough assay and one assay at any other time will allow DoseMeRx to accurately calculate AUC.
Authors’ Highlight:
Authors’ Highlight:
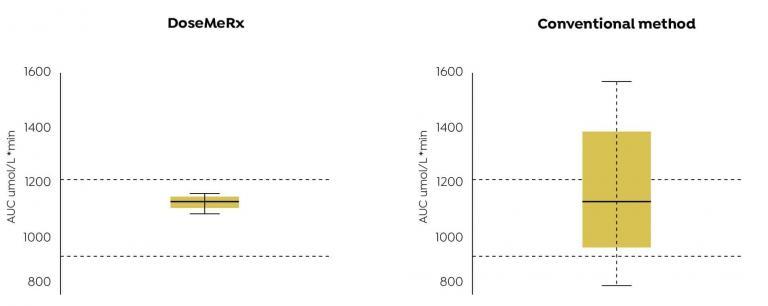
AUCs achieved using DoseMeRx with two assay results were more likely to fall within the therapeutic range than that achieved by adjusting the dose using the conventional method.
BENEFITS OF DOSEMERX FOR TDM OF BUSULFAN
CONCLUSION
This study demonstrates that busulfan TDM in HCT patients can be simplified safely using DoseMeRx with only two samples. The reduced requirement for blood collection samples not only lessens the distress to HCT patients, but also greatly reduces the cost by decreasing unnecessary assays. It also permits for more frequent dose adjustment allowing for changes in individual patient’s pharmacokinetics.
Overall, this study shows that DoseMeRx reduces the requirement for multiple blood sample collection and assay processing. By allowing for a wide range of times at which the two assays may be sampled, DoseMeRx
reduces the likelihood of inappropriate use of assays. This assists healthcare practitioners to select the most appropriate dose to achieve an optimal therapeutic outcome and reduce potential toxicity.

DoseMeRx using a two-sample method is able to accurately calculate an individualised dose to achieve Busulfan exposure in therapeutic range using only onequarter of the laboratory results required by the conventional method.
Using the DoseMeRx, only two assays results are required for accurate AUC estimation.

DoseMeRx using a two-sample method allows less frequent collection at a more relaxed time regime. While ideally the samples should be collected 2.5 and 6 hours after administration, any combination of two samples that include the trough is as accurate as conventional methods.
Try DoseMeRx – Free Trial
Try DoseMeRx for yourself, with a free 14 day trial including your choice of drug packages from a wide range of medications, customize dose or target functions to individualize treatment for every patient, and access our mobile and tablet apps for iOS and Android. During your free trial, you’ll also have access to our support team who are here to answer any questions you have. Start free trial now >>
AUTHORS
Paul E. Sabourenkov, MBioInf (Adv), Robert C. McLeay, PhD.
REFERENCES
1. Bartelink, I. H., Lalmohamed, A., van Reij, E. M. L., Dvorak, C. C., Savic, R. M., Zwaveling, J., et al.
(2016). Association of busulfan exposure with survival and toxicity after haemopoietic cell
transplantation in children and young adults: a multicentre, retrospective cohort analysis.
The Lancet Haematology, 3(11), e526–e536. http://doi.org/10.1016/S2352-3026(16)30114-4
2. Lombardi, L. R., Kanakry, C. G., Zahurak, M., Durakovic, N., Bolaños-Meade, J., Kasamon, Y. L., et al. (2015). Therapeutic drug monitoring for either oral or intravenous busulfan when combined with pre- and post-transplantation cyclophosphamide. Leukemia & Lymphoma, 57(3), 666–675. http:// doi.org/10.3109/10428194.2015.1071488
3. Salman, B., Al-Za’abi, M., Al-Huneini, M., Dennison, D., Al-Rawas, A., Al-Kindi, S., et al. (2017). Therapeutic drug monitoring-guided dosing of busulfan differs from weight-based dosing in
hematopoietic stem cell transplant patients. Hematology/Oncology and Stem Cell Therapy, 10(2), 70–78. http://doi.org/10.1016/j.hemonc.2017.03.003
4. Sandström, M., Karlsson, M. O., Ljungman, P., Hassan, Z., Jonsson, E. N., Nilsson, C., et al. (2001). Population pharmacokinetic analysis resulting in a tool for dose individualization of busulphan in bone marrow transplantation recipients. Bone Marrow Transplantation, 28(7), 657–664. http:// doi.org/10.1038/sj.bmt.1703229
5. Hennig S et al. Clinical Pharmacokinetics 2013; 52:289-301.8. Li C, Therapeutic Drug Monitoring of Vancomycin in the real world. IATDMCT Compass. Mar 2017.
6. Li C, Therapeutic Drug Monitoring of Vancomycin in the real world. IATDMCT Compass. Mar 2017
