Optimizing Vancomycin AUC Dosing in Vascular Surgery Patients at University Hospitals of Leicester NHS Trust
In this article:
Key Findings
- DoseMeRx improved the overall accuracy and precision of vancomycin dosing in patients with vascular infections.
- Significantly fewer patients missed vancomycin doses in the DoseMeRx group, which can have direct implications on improving medication safety.
- The use of DoseMeRx resulted in a significantly higher proportion of patients who reached the therapeutic AUC24 target, leading to improved pharmacodynamic outcomes in patients treated with vancomycin.
Executive Summary
Vancomycin is a widely used antibiotic for gram-positive infections. While therapeutic drug monitoring of vancomycin blood levels is a longstanding practice, a shift has occurred over the past several years from trough-based monitoring to area-under-the-curve-based monitoring. This poses new challenges to clinicians, as the measurement and calculation of area under the curve (AUC) is less straightforward than evaluating trough levels.
One solution to those challenges is individualized dosing via Bayesian decision support software, which is the preferred approach recommended by the 2020 vancomycin dosing guidelines.
The study performed at Glenfield Hospital, University Hospitals of Leicester NHS Trust, and published in the British Journal of Clinical Pharmacology, compared vancomycin AUC outcomes in vascular surgery patients managed with DoseMeRx, a Bayesian decision support tool, to patients managed by traditional trough-based dosing methods.(1)
The Problem
Vancomycin is a glycopeptide antibiotic used for gram-positive bacterial infections. Due to the narrow therapeutic index and potential toxicities of vancomycin, use of this drug involves therapeutic drug monitoring (TDM) of vancomycin blood levels.
While AUC has long been accepted as the pharmacodynamic endpoint for vancomycin, and until recently, measurement of a vancomycin trough was accepted as a surrogate marker for AUC exposure. However, given increasing data indicating that trough is not a predictable surrogate for AUC, the 2020 vancomycin dosing guidelines (endorsed by IDSA, SIDP, ASHP, and PIDS)a recommend monitoring AUC over trough.(2)
Issues in Conventional Dosing of Vancomycin
The shift from trough-based to AUC-based vancomycin dosing grew from the increasing body of evidence that vancomycin troughs prove to be an unreliable marker of AUC exposure, often resulting in patients receiving higher-than-necessary doses and, subsequently, increased toxicity. Counterintuitively, pharmacokinetic studies suggest that some patients may also have sub-therapeutic AUC values at therapeutic or supra-therapeutic trough values, further highlighting the discontinuity between AUC exposure and trough values. Additionally, clinical studies demonstrate improved patient outcomes when AUC is maintained in target range in infections with methicillin- resistant Staphylococcus aureus and Enterococcus species.(3,4,5)
Current Challenges Associated with Vancomycin Dosing
There are many challenges to implementing AUC- guided vancomycin dosing. Unlike trough values, AUC exposure cannot be measured directly from a laboratory value viewed in the medical record – it must be calculated either with pharmacokinetic equations or with software support. Additionally, as vancomycin dosing has been a paradigm of clinical pharmacy for decades, the cognitive shift from trough-based vancomycin dosing to AUC-guided dosing can prove a difficult transition. Finally, without software assistance such as DoseMeRx, AUC measurement involves waiting until the patient is at steady state, then drawing two blood levels at fixed times. These levels are relative to the timing of the dose, which can often result in missed or delayed doses and the need for repeat blood draws.
Clinical Relevance
The guideline supports two methodologies for AUC monitoring: traditional PK modeling with multiple blood/serum levels and Bayesian kinetic modeling with single or multiple blood/serum levels.(2) The present study assesses AUC outcomes in patients using the Bayesian method as highlighted in the 2020 guidelines utilizing DoseMeRx.
The Role of Missed Doses on Medication Safety
A medication that is ordered but not dispensed or administered to the patient is classified as a medication error. Reducing medication errors is a global goal in healthcare that is recognized by Regulatory, Quality and Accreditation organizations.(6, 7)
A missed dose of a medication can have detrimental impacts on a patient’s care both in and outside of the hospital. For example, in patients who are being treated for an infectious disease, such as a vascular surgery infection, a missed dose of the treating antibiotic can result in a worsening of the infection or could increase the potential for antimicrobial resistance to occur due to suboptimal concentrations, both in the blood, but also at the site of infection. This case study highlights the impact that utilizing DoseMeRx had on reducing the number of missed doses, thus having a direct impact on improving the safe use of medications.
Outline of the Study
Aim
The aim of this observational cohort study was to assess the outcomes related to the implementation of bed-side, Bayesian-guided vancomycin dosing using DoseMeRx.
Design
The study cohort consisted of vascular surgery patients admitted to Glenfield Hospital, University Hospitals of Leicester NHS Trust. Prior to initiation of the study, physicians and pharmacists were given access to DoseMeRx and trained on use of a clinically-validated, one-compartment vancomycin dosing model. Vancomycin dosing was primarily managed
by clinical pharmacy staff, but physicians managed vancomycin dosing independently on the weekends. Vancomycin levels were typically drawn pre-steady-state, between the loading dose and the first maintenance dose.
The DoseMeRx group prospectively enrolled patients between June 26, 2018 and February 28, 2019 who were prescribed intravenous vancomycin and were not on hemodialysis. The most common indication for antibiotics in the both groups was diabetic foot infection, and the most commonly co-administered antibiotics were meropenem and piperacillin/ tazobactam. The prospective group was compared against a similar retrospective control group for whom vancomycin was not dosed via DoseMeRx.
Primary outcome
The primary outcome of the study was 24-hour AUC (AUC24) value for each group. For the prospective group, AUC24 values were determined using DoseMeRx. For the control group, laboratory values and vancomycin dosing regimens were retrospectively entered into DoseMeRx to assess AUC24 exposure. From the AUC24 data, mean AUC24 exposure and percentage time in therapeutic range (%TTR) were calculated.
Secondary outcomes
Secondary outcomes included:
- acute kidney injury (AKI),
- all-cause 30-day mortality,
- amputation rates,
- re-admission rates
- number of missed doses.
It is important to note that the target AUC range in this study (350-450 mg/L*h) was slightly lower than the range espoused by the 2020 vancomycin dosing guidelines (400-600 mg/L*h).
Results
- Vancomycin levels predicted by DoseMeRx had a high correlation with measured vancomycin levels (r2= 0.86).
- The average number of missed doses was five times lower in the DoseMeRx group than in the control group (0.23 doses per course vs 1.04 doses per course, respectively; p<0.00001).
- A significantly higher percentage of patients in the DoseMeRx group achieved an average 24 hour AUC within the goal range used in the study when compared to the control group (68.3% vs 41.7%, p = 0.005).
In the study, 104 patients were enrolled in the DoseMeRx group and 139 patients were included in the retrospective control group. The two study arms were well matched (Table 1), with the exception that meropenem was more commonly used as a concomitant antibiotic in the control group and piperacillin/tazobactam was more commonly used in the DoseMeRx group.
The study achieved its primary outcome. Researchers found a statistically significant higher proportion of patients in the DoseMeRx group achieved mean AUC24 values within goal range as compared to the control group. In addition, the number of doses that were outside of therapeutic AUC24 range were significantly higher in the control group (p<0.005).
There were no differences in the clinically related secondary outcomes measured, but a reduction in missed doses was seen (Figure 1).
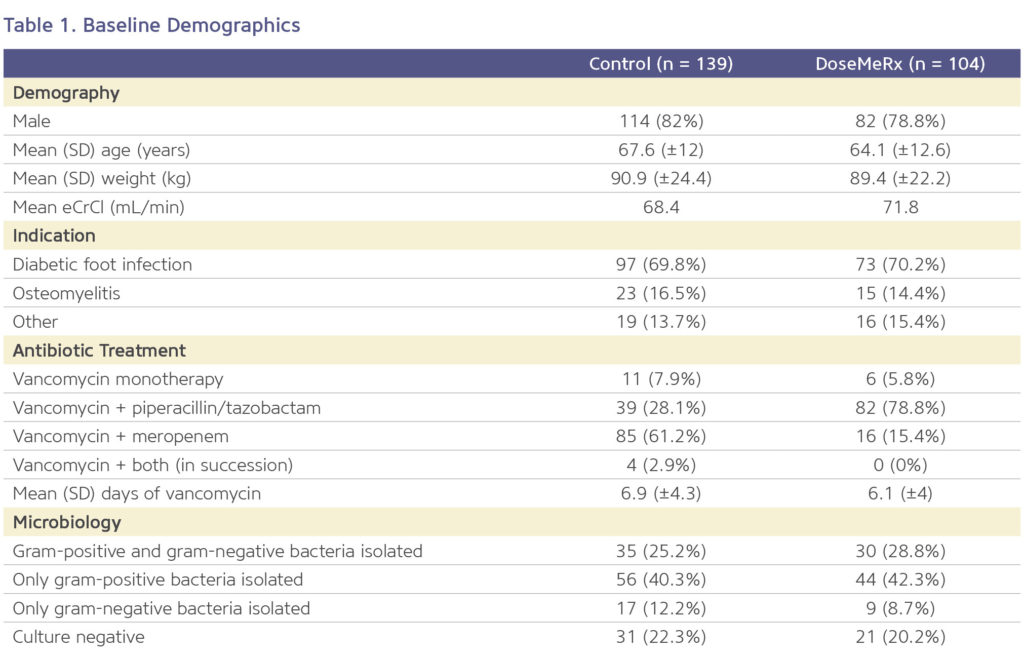
Improving Medication Safety By Reducing Missed Doses
Bayesian guided dosing resulted in fewer missed doses of vancomycin (Figure 1). DoseMeRx can interpret pre-steady state vancomycin levels and those levels drawn outside of the normally scheduled time.
This flexibility is beneficial because it eliminates delays or omissions of doses that may occur when having to wait for a vancomycin level to be drawn at a specific time of day or point in the patient’s course.
Predictive Performance of DoseMeRx
The study compared the difference between the levels that were predicted by DoseMeRx vancomycin model with the actual measured levels. Across all patients, there was a strong correlation between what was estimated by DoseMeRx and the vancomycin levels reported by the Lab.
The predictive performance of DoseMeRx, as assessed by comparing observed concentrations and model predicted values, showed good correlation (r2= 0.86).
The average of absolute difference between measured and predicted concentration was 1.57mg/L for patients in the control group vs. 1.48 mg/L for DoseMeRx.
DoseMeRx Bayesian Dose Forecasting
Bayesian dosing combines pharmacokinetic modeling with statistical probabilities to provide individualized dosing for patients. Bayesian models take into account readily available patient information – such as height, weight, renal function, and drug levels – and estimate a patient’s ability to absorb, process, and clear a drug from their system. When a patient is initiated on a drug, the pharmacokinetic model will be largely derived from population data; however, as more data is entered, a truly individualized dosing model develops.
While Bayesian dose forecasting is a long-established clinical tool, its use was previously restricted to clinicians who were also experienced with statistics and information technology. DoseMeRx provides the clinical benefits of Bayesian dose forecasting plus a user-friendly interface that is accessible to the average clinician. All of this, along with the training and support offered by DoseMeRx, make bringing the benefits of Bayesian dosing to the bedside easier than ever before.
While Bayesian dose forecasting is a long-established clinical tool, its use was previously restricted to clinicians who were also experienced with statistics and information technology. DoseMeRx provides the clinical benefits of Bayesian dose forecasting plus a user-friendly interface that is accessible to the average clinician. All of this, along with the training and support offered by DoseMeRx, make bringing the benefits of Bayesian dosing to the bedside easier than ever before.
DoseMeRx makes bringing the benefits of Bayesian dosing to the bedside easier than ever before.
References
1 Vali L et al. Br J Clin Pharmacol; 2020: 1– 10.
2 Rybak M et al. Am J Health-Syst Pharm; 2020: 7(11):835-864.
3 Brown J et al. AAC;2012, 56 (2): 634-638.
4 Jumah MT et al. AAC: 2018:62(3).
5 Mogle B et al. Int J Antimicrob Agents; 2018: 52(6):805-810.
6 https://www.nccmerp.org/about-medication-errors
7 The Joint Commission (https://www.jointcommission.org/), National Health Services (https://www.england.nhs.uk/patient-safety/national-medicines-safety-programme/), FDA (https://www.fda.gov/drugs/drug-information-consumers/working-reduce-medication-errors), Leapfrog (https://www.leapfroggroup.org/ratings-reports)

Download the Optimizing Vancomycin AUC Dosing at University Hospitals of Leicester NHS Trust Case Study
Richiedi una dimostrazione di prova
Scopri quanto è facile usare e integrare DoseMeRx nel tuo lavoro quotidiano.
Richiedi una dimostrazione di prova qui sotto. Puoi anche chiamarci al numero +1 (832) 358-3308 oppure scriverci all’indirizzo hello@dosemehealth.com
"*" indica i campi obbligatori
