Vancomycin Loading Dose: The Pharmacokinetics to Consider
In this article:
Note: The new vancomycin dosing guidelines have been officially released. Learn what’s changed and how they’ll impact you by reading our guide to the new vancomycin dosing changes here.
A vancomycin loading dose – a large dose given once at the onset of therapy to rapidly achieve a therapeutic drug concentration – is required due to the pharmacokinetics of vancomycin to ensure that vancomycin levels are not subtherapeutic at the beginning of treatment.
Vancomycin is a broad spectrum glycopeptide antibiotic commonly prescribed for infections caused by gram-positive bacteria:1
- pneumonia
- pleural empyema
- infective endocarditis
- colitis associated with Clostridium difficile
- enterocolitis
- cellulitis
- meningitis
- bacteremia
- sepsis
Vancomycin exhibits antibacterial activity by disrupting the peptide structure of the bacterial cell wall during the synthesis process.2
The pharmacokinetics of vancomycin
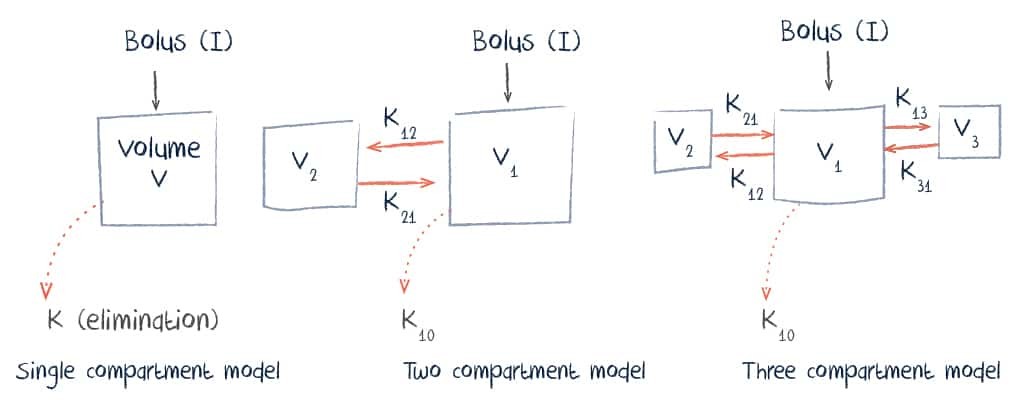
Pharmacokinetically, vancomycin can be described using either a single, two or three compartment model.
Administration, Distribution and Excretion of Vancomycin
Vancomycin is normally given intravenously. Oral absorption is limited and this route is only useful for infections of the gastrointestinal tract.
With intravenous infusion, distribution typically occurs over a period of about 30 minutes and volume of distribution is between 0.4 and 1 L/kg. This is influenced by numerous factors including body mass index, pregnancy, and the presence of inflammation.
Vancomycin is eliminated renally with 80-90% excreted unchanged in the urine.
Pharmacodynamics of Vancomycin
The bacteriostatic activity of vancomycin is time dependent, an AUC24:MIC target of approximately 400mg.h/L is recommended as best correlating with clinical outcomes. Due to the complexity of calculating area under the curve (AUC) without software such as a DoseMeRx, it is often clinical practice to monitor serum trough levels (with an assumed MIC of 1 for Staphylococcus aureus).4 This varies based on patient, infection type and, bacterial pathogen although, in most cases, a trough level of 10-20 mg/L is targeted.
Calculating a Vancomycin Dose
As bacteriostatic activity is not concentration-dependent, dosing is based achieving the target trough level for a given patient. The appropriate dose for a patient may be calculated based on their weight and the volume of distribution for vancomycin.
Dosing is generally based on actual body weight rather than ideal body weight.5 Dosing calculators such as DoseMeRx are useful clinical tools to individualize each dose. Many clinical references provide suggested dosing ranges for patients based on infection type.
Why administer a vancomycin loading dose?
Due to the pharmacokinetics of vancomycin, a loading dose – a large dose given once at the onset of therapy to rapidly achieve a therapeutic drug concentration in the body – is required to ensure that vancomycin levels are not subtherapeutic at the beginning of treatment.6,7
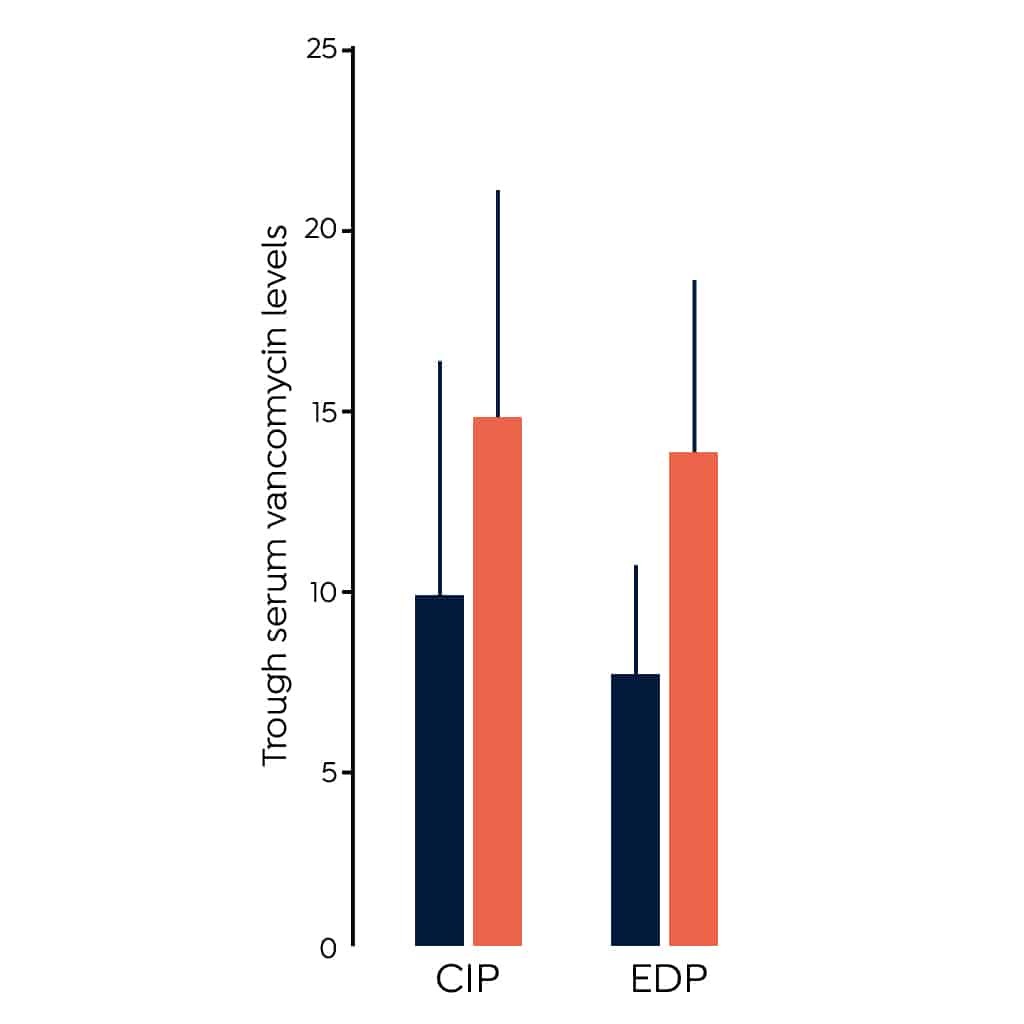
- A 2011 randomized clinical trial (RCT) indicated a loading dose of 25-30 mg/kg leads to higher peak levels than initiating therapy without a loading dose in critically ill patients (CIP).3 This study measured peak concentration which is not a good measure of clinical efficacy, additional studies have contributed to evidence supporting the use of a loading dose.
- A small RCT of 99 emergency department patients (EDP) treated vancomycin indicated a higher percentage of those given a loading dose of 30 mg/kg achieved trough levels > 15 mg/L at 12 hours post therapy initiation than those treated with the standard therapy of 15 mg/kg.8The figure above shows the difference in trough levels of the patients given a loading dose compared to those given standard therapy in these two studies.
- A third study found no difference in the proportion of patients treated with a fixed 2g loading dose and the proportion of patients treated with standard therapy who achieved a trough level > 15 mg/L. This study included only 11 patients and therefore sample size is a major limitation.9
Vancomycin Administration
Vancomycin is normally administered intravenously due to low oral bioavailability.3 Occasionally the rectal route is used for gastrointestinal infections.1
Doses are given every 8 to 12 hours.4 If other antibiotics are being given simultaneously, administration should be timed to avoid potential drug interactions or incompatibilities. A standard infusion rate of 1 g over 60 minutes is commonly used and the infusion duration varies based on the quantity of drug required. The infusion rate should not be increased above this rate due to the risk of the development of “red man syndrome,” an infusion-related reaction that causes an erythematous rash to develop on the upper body and face.10
Laboratory and Clinical Monitoring of Vancomycin
Serum concentration is normally collected at steady state trough which usually occurs prior to the administration of the fourth dose.4 Peak concentration has not been shown to correlate to either toxicity or efficacy and is therefore usually not monitored.11
Clinically, symptoms of infection should be assessed regularly. These include body temperature and heart rate. Blood cell counts can also be helpful markers of infection improvement or worsening.
Vancomycin Use in Pediatrics
Vancomycin is an antibiotic commonly used in children with severe infections – a common one being sepsis. Pharmacokinetic parameters in pediatrics vary from those expected in adults and, for this reason, doses should be calculated carefully.
Children should be monitored closely to ensure that they are responding well to vancomycin therapy, both in terms of infection resolution and development of any side effects. Used correctly, this medication can be a great option for seriously ill pediatric patients.
Safety and Side Effects of Vancomycin
Vancomycin Induced Nephrotoxicity
A risk of nephrotoxicity has been linked to vancomycin. Concern for the development of this side effect has made some clinicians reluctant to adopt the practice of initiating therapy with a loading dose.
In the above-described trial of 99 patients, no difference in the incidence of nephrotoxicity was observed between the treatment and control groups.8 The authors do note this study was not appropriately powered to detect such a difference and results should be interpreted cautiously. An additional retrospective cohort study of 1,330 patients also investigated the effect of vancomycin dose on the development of kidney injury.12 Fewer patients in the high-dose group developed renal adverse events than did low-dose patients. The study further demonstrated that the relative risk of acute kidney injury was also greater in the high-dose group.
While safety concerns should lead healthcare professionals to closely monitor vancomycin patients, in most cases, these risks should not preclude use.
Try DoseMeRx – Free Trial
Try DoseMeRx for yourself, with a free 14 day trial including your choice of drug packages from a wide range of medications, customize dose or target functions to individualize treatment for every patient, and access our mobile and tablet apps for iOS and Android. During your free trial, you’ll also have access to our support team who are here to answer any questions you have. Start free trial now >>
References
- http://www.pdr.net/drug-summary/Vancocin-vancomycin-hydrochloride-802
- http://www.clinicalpharmacology-ip.com/Forms/login.aspx?ReturnUrl=%2fForms%2fMonograph%2fmonograph.aspx%3fcpnum%3d638%26sec%3dmonmech&cpnum=638&sec=monmech
- https://academic.oup.com/cid/article/42/Supplement_1/S35/275535
- http://www.ajhp.org/content/66/1/82
- http://med.stanford.edu/content/dam/sm/bugsanddrugs/documents/dosing/2013VancEDloadingdose_000.pdf
- https://www.merriam-webster.com/medical/loading%20dose
- http://aac.asm.org/content/60/5/2601.full
- http://journals.sagepub.com/doi/abs/10.1177/1060028014556813
- https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1445-5994.2011.02459.x
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC270616/
- https://www.karger.com/Article/Abstract/343162
- https://academic.oup.com/cid/article/63/1/iii/1745274
Related Posts
Here are some more vancomycin resources for you: