Vancomycin Dosing Therapeutic Guideline 2019: What’s Changing?
In this article:
Note: The new vancomycin dosing guidelines have been officially released. Learn what’s changed and how they’ll impact you by reading our guide to the new vancomycin dosing changes here.
The draft of the revised vancomycin therapeutic guidelines by Rybak et al. for the Infectious Diseases Society of America (and the ASHP, PIDS, and SIDP) are now available for public comment.
The update, as many expected, recommends moving away from trough targets to AUC24:MIC for the monitoring of vancomycin. Although trough-only therapeutic monitoring for vancomycin is widely used today in clinical practice due to the ease of use, these guidelines present AUC24/MIC to be the most appropriate pharmacokinetic/pharmacodynamic target for vancomycin.
This shift, in part, may have been promoted due to recent studies that have demonstrated there is significant nephrotoxicity risk when targeting higher vancomycin trough concentrations. The research suggests for many patients, an effective AUC24:MIC target of 400-600 mg.h/L can be achieved with significantly lower trough concentrations than previously recommended.
Highlights:
- An AUC24 of 400-600 mg.h/L is the recommended target to “achieve clinical efficacy while improving patient safety”. Trough targets are “no longer recommended for patients with serious infections due to MRSA”.
- AUC is the driver of effectiveness and the risk of acute kidney injury (AKI) is related to trough concentration.
- Bayesian dosing is the recommended method for dosing vancomycin. Bayesian dosing is shown in the guidelines to require only half the levels of other manual approaches. “Bayesian programs offer numerous advantages over traditional first-order software…”
- Acute kidney injury occurs in between 5-43% of patients, with an attributable risk to vancomycin of 59%. Even mild AKI can “significantly decrease long-term survival rates, increase morbidity, prolong hospitalizations, and escalate healthcare costs.”
Vancomycin AUC Conversion Toolkit
Get instant access to our concise range of free checklists, tips and templates to support you in successfully transitioning to an AUC-based dosing strategy.
When managing vancomycin therapy for diverse cohorts including obese and hemodialysis, it is recognized that there is significant pharmacokinetic variability both between and within these groups. The recommendations for use of Bayesian dosing for individualized dosing in these cohorts is also discussed at length in these guidelines.
Calculating Area Under the Curve – Pharmacokinetics Refresher
Understanding some pharmacokinetics is important to understand how monitoring can be performed for dosing to an Area Under the Curve (AUC) target. Some terms to be familiar with in order to understand the concept of AUC are:
- Half-life (t1/2): The time needed for half of the amount of drug present in the bloodstream to be removed. The half-life will be different for each drug depending on how fast it’s eliminated from the body.
- Cmax: The peak concentration of drug.
- Cmin: The trough concentration of drug.
- AUC: The Area Under the Curve is defined as the total amount of drug that is in the bloodstream over a period of time. Literally, it is the area under the curve between two points on the plot (for mathematicians, the definite integral of the concentration-time profile). It is sometimes described as “total exposure to the drug” within a certain time-window.
- Steady-state: When the rate of drug intake matches drug elimination. I.e. a consistent amount of the target drug in the bloodstream remains even though dosing continues. This occurs after a certain period of time dependent on the drug and patient. The peaks and troughs will look similar when steady state has been reached – typically after 4 to 5 half-lives.
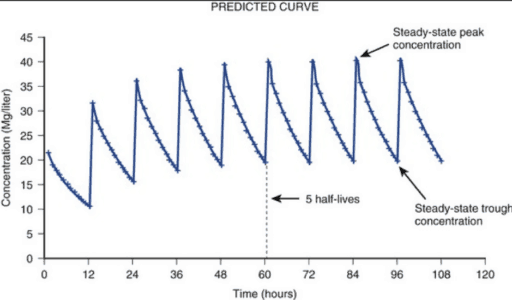
The minimum inhibitory concentration (MIC)is the minimum amount (dose) of the antimicrobial drug needed to inhibit the visible growth of bacteria. There are different values for MIC depending on what type of antibacterial drug you use and how resistant a bacteria is to a specific antimicrobial drug. For example, a MIC=1mg/L indicates that the visible growth of bacteria is inhibited from 1mg/L of the drug. Typically, the AUC will have to be divided by the MIC (known as AUC/MIC ratio) to take into account how much of the drug is needed over a period of time (the AUC) to inhibit a certain bacteria 100 percent.
All of these concepts permit the calculation of AUC by hand using a set of formulae. Unfortunately, as one might expect, this is a rather lengthy process. A recent publication by Meng et al.(2019) developed and implemented an abbreviated calculation version that still had 15 separate steps with multiple drug levels!
Problematically, however, most hand-calculation methods assume that the patient is already at steady-state. This has the consequence whereby if one calculates dose adjustments manually, levels cannot be taken to permit this to be done until day 3 of therapy at the earliest. Even more problematically, the guidelines recommend that “targeted AUC exposures be achieved early within the course of therapy, preferably within the first 24 to 48 hours”.
Vancomycin dosing and monitoring AUC – Wait until Steady-state?
One of the major benefits of Bayesian dosing indicated by the revised guidelines is Bayesian dosing “doesn’t require steady-state vancomycin concentrations to allow to early assessment of AUC target attainment”.
In other words, if you use Bayesian dosing, you can take a single level after the first dose is administered, and dose adjust immediately. While this method requires software, it is obviously much faster to calculate (e.g. than the 15-step ‘abbreviated’ method), and quicker to adjust for the patient.
Bayesian dosing is also noted to be effective from a single level, although multiple levels (one shortly after the end of infusion, and one at the end of the dosing interval) is a preferred option if possible.
Hang on, what about the MIC part in “AUC24:MIC”?
Typically, most centers will take approximately three days to return microbiology results, including the MIC. So what approach do the new 2019 guidelines suggest until then?
Of course, an AUC for vancomycin depends on the bacteria that’s being treated. Given vancomycin’s common use, it is noted in the guidelines that “it is important to be aware of local and national vancomycin susceptibility patterns for MRSA”. That point made, for methicillin resistant staphylococcus aureus (MRSA) it is also noted that vancomycin MICs have remained constant over time, with ≥90% of isolates having MIC < 1mg/L.
Given this, the guidelines suggest that an appropriate level of AUC over 24 hours should be 400-600 mg*hr/L with an assumed minimum inhibitory concentration of 1 mg/L if this is not yet known.
For those interested in the laboratory side, the MIC method cited in the new guidelines is broth micro-dilution (BMD). The discussion around the variance between BMD results and ETests on page 10 of the draft 2019 guidelines is worthwhile considering.
The Impact of AUC on Vancomycin Dosing
Moving away from a single lab-reported number in a range (trough) to using a calculated number (AUC) instead may appear somewhat challenging at first glance. That said, the guidelines do provide for flexibility – for example, noting that while two levels on a single dose are optimal, using Bayesian software permits the use of a single trough level – i.e. no nursing or phlebotomy workflow changes required.
One of the key benefits of DoseMeRx (our Bayesian dosing platform) is that it is configured to match each hospital’s protocols and requirements. DoseMeRx is used to dose vancomycin to both trough and AUC-based targets.
Regardless of your plans for uptake of the new vancomycin guidelines, it can support your dosing protocol. For example, many of our customers have DoseMeRx configured to dose to trough now, but also display AUC24 side-by-side – preparing and familiarizing their pharmacy staff for a future rollout of AUC-based vancomycin dosing.
Learn more
Read more on vancomycin dosing and AUC calculations using our bayesian dosing calculator. You can also learn about vancomycin trough levels here.
Sources
- Rybak, MJ. Le J Lodise LP; et al. “Therapeutic Monitoring of Vancomycin: A Revised Consensus Guideline.”.
- Doogue, Matthew. Polasek Thomas. Therapeutic Advances in Drug Safety. “The ABCD of Clinical Pharmacokinetics.” Published February 2013.
- Andrews, JM. Determination of minimum inhibitory concentrations. Published Jul 2001
- https://www.ncbi.nlm.nih.gov/pubmed/11420333
- Anderson, Peter. TheBody. The ABCs of Pharmacokinetics.
- Holmes, Natasha.Turnidge John. Munckhof Wendy; et,al. American Society for Microbiology. “Vancomycin AUC/MIC Ratio and 30-Day Mortality in Patients with Staphylococcus aureus Bacteremia.”
- University Health System. “Vancomycin Dosing for Adults.”
- American Journal of Health System Pharmacists. “Therapeutic Monitoring of Vancomycin in Adult Patients: “A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists.” Published 2009
- Czosnowski,Quinn. Miano Todd. Clingate. “Principles of Drug Dosing in Critically Ill Patients.”
Request a demo
See how easy DoseMeRx is to operate and integrate into your workday.
Request a demo below. You can also phone us on +1 (832) 358-3308 or email hello@dosemehealth.com.
"*" indicates required fields
